The Critical Role of Magnesium Ions in DNA
Polymerase 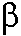 's Closing and Active Site Assembly
's Closing and Active Site Assembly
To dissect the effects of the nucleotide-binding and catalytic metal ions
on DNA polymerase mechanisms for DNA repair and synthesis, aside from the
chemical reaction, we investigate their roles in the conformational
transitions between closed and open states and assembly/disassembly of the
active site of a pol beta/DNA complex before and after the chemical
step of nucleotide incorporation. Using dynamics simulations, we find that
closing before chemistry requires both divalent metal ions in the
active site while opening after chemistry is triggered by release of
the catalytic metal ion. The critical closing before chemistry is
stabilized by the interaction of the incoming nucleotide with conserved
catalytic residues (Asp190, Asp192, Asp256) and the two functional
magnesium ions; without the catalytic ion, other protein residues (Arg180,
Arg183, Gly189) coordinate the incomer's triphosphate group through the
nucleotide-binding ion. Because we also note microionic heterogeneity near
the active site -- Mg2+ and Na+ ions can diffuse into the active
site relatively rapidly -- we suggest that the binding of the catalytic
Mg2+ itself is not a rate-limiting conformational or overall step.
However, geometric adjustments associated with ion and proper positioning
in the active site, including subtle but systematic motions of protein
side chains (e.g., Asp192, Arg258), define slow or rate-limiting
conformational steps that may guide fidelity mechanisms. These sequential
rearrangements are likely sensitively affected when an incorrect
nucleotide approaches the active site. Our suggestion -- that subtle and
slow adjustments of the nucleotide-binding and catalytic magnesium ions
help guide polymerase selection for the correct nucleotide -- extends
descriptions of polymerase pathways and underscores the importance of the
delicate conformational events both before and after the
chemical reaction to polymerase efficiency and fidelity mechanisms.

 Click to go back to the publication list
Click to go back to the publication list
